Arquitos Capital's commentary for the year ended December 31, 2025.
Dear Partner:
Arquitos returned 82.1% net of fees and expenses in 2025, compared to 12.8% for the Russell 2000. Individual returns will vary based on timing of investment. Please check your statement for specific results.
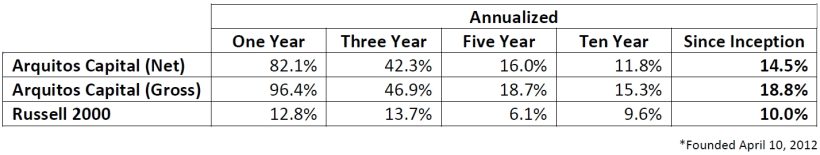
In 2025, Arquitos Capital completed its best year in its 14-year history. We have returned higher than 40% in six of those 14 years.
One year ago, I wrote that I was as confident in the portfolio as I have ever been. While I am pleased by the performance last year, I also believe that our portfolio companies may be just as undervalued today as they were one year ago.
We are primarily concentrated in our top three positions: Liquidia Corporation, ENDI Corp, and Finch Therapeutics. We also have a handful of small positions and some cash. I have been looking at a variety of potential new investments in order to diversify the portfolio, but the good news is that I have not found any that offer a more compelling risk/reward profile than our current holdings.
As a reminder, Arquitos is an unconventional hedge fund invested in a small number of unique companies. I look for company-specific situations where the potential reward meaningfully outweighs the risk. I want multi-bagger potential with limited downside risk.
I am looking for situations where there is a misunderstanding by the market. Typically, this occurs in companies that are going through transitions. There may be structural or strategic changes within the company including reorganizations, spin-offs or some other catalyst that is being unlocked. Significant misunderstandings happen more often in smaller companies, so most of my focus is in that area.
These opportunities are fairly rare. That is why the portfolio is concentrated. It can sometimes take years for the public to understand what is happening at the company, or for the transition to fully occur. Because of that, short-term performance is not particularly relevant. A company can trade for a depressed price for an extended time period and then have its stock price dramatically increase over a short amount of time once the markets have more clarity.
I have written about this in the past and feel very strongly that we have a significant advantage being comfortable with uncertainty, if the downside is protected by a strong balance sheet. Investors love certainty and will pay a premium for it. But often we have opportunities where extremely positive things are happening at a company behind the scenes, but it takes several quarters or years for the income statement to show it. By investing early in those situations, we can capture tremendous upside prior to other shareholders buying in. Then, we can get another leg up in the stock price when those new, more income statement-oriented investors enter.
I have applied this strategy to our three largest positions below:
Liquidia Corporation (LQDA)
Liquidia was our largest contributor in 2025. Shares ended the year at $34.49, increasing from $11.76 at the start of the year. We primarily own long dated call options that are in the money.
We first bought Liquidia in October 2022 at $4.83 per share. At the time, the company was solely a legal special situation play. Prior to our entry, Liquidia had lost a case at the federal district court level involving the ‘793 patent owned by competitor, United Therapeutics (UTC). However, the Patent Trial and Appeals Board (PTAB) then found that the ‘793 patent was invalid. This district court decision had been handed down prior to the PTAB decision.
This created an interesting dynamic and extremely attractive entry point. The next step was a PTAB appeal, which Liquidia won. Then, of course, the district court decision had to be overturned since you cannot violate a patent that is not valid.
Since then, we have had several other legal impediments involving Liquidia, UTC, and the Federal Drug Administration. Eventually, in late May 2025, Liquidia’s medication, Yutrepia, finally came to market.
This situation was a sweet spot for the Arquitos strategy. My ideal situation is entering an investment that is a special situation. Due to the uncertainty and complexity, there is a higher chance that the stock is mispriced. Then, when the special situation aspect is resolved and the company performs operationally, a new set of investors who are focused on the income statement begin to invest. This transition involving turnover into different types of investors can create significant opportunities.
Strict special situation investors exit with a nice gain after the legal situation is done, but they miss out on the additional gains (and potential future buy-out) that come later. Income statement or growth investors buy from the special situation investors once there is more clarity. These investors missed out on the initial gains but benefit from the longer-term operational success of a company.
My ideal situation is to capture both the special situation gains and the longer-term gains.
Liquidia fit this approach well. Their launch may go down as one of the most successful drug launches ever. They have already taken 25% market share and are effectively expanding the market. Amazingly, 75% of all patients starting on Yutrepia are new to treprostinil.
As a reminder, treprostinil is the generic drug delivered either through inhaled methods, oral, via IV or subcutaneous. Liquidia’s treatment method is more effective than their competitor because of Liquidia’s patented PRINT manufacturing process. PRINT allows the treprostinil powder to be smaller and in a more uniform shape, allowing the drug to reach the deep lung in patients. This both increases effectiveness and reduces side effects like cough. Many patients have a very difficult time breathing, and Yutrepia’s low effort inhaler is much easier for them. The competitor’s manufacturing process does not allow for the use of a low effort inhaler.
Other delivery methods for treprostinil carry their own side effects and are used at different stages of disease progression. It is generally better for the patient to be on the inhaled treatment as long as side effects are lower and high enough doses of treprostinil can be delivered to the deep lung. This is why clinical studies involving Yutrepia show how effective it is for patients. Yutrepia can deliver higher doses with fewer side effects. Liquidia should be able to capture, and already are capturing, the inhaled market, naïve patient market, and some of those patients on other treatment regimens.
Specific to the inhaled market, CEO Roger Jeffs believes that Liquidia can take 80% to 90% of UTC’s market share. Liquidia’s Q4 results show that this is beginning to happen. Liquidia generated $30 million in positive free cash flow in Q4 2025 on $90 million in revenue. That number will exponentially rise from here.
The final significant legal issue is a case where UTC alleged that Liquidia violated UTC’s ‘327 patent. That patent generally covers improving exercise capacity in patients suffering from PH-ILD. This patent and case do not involve the treatment of patients suffering from PAH.
The trial was in late June 2025. We are expecting a decision any day. The outcome could range from a full win by Liquidia to a full removal of Liquidia’s ability to have PH-ILD listed on Yutrepia’s labeling. There also could be potential outcomes in between such as some sort of royalty involving PH-ILD prescriptions. Liquidia is confident in a full win, but anything can happen in litigation. Many legal observers believe that the longer it has taken for the judge to provide his decision, the better it is for Liquidia.
Importantly, Liquidia has stated that 75% of their revenue so far is from PAH prescriptions. So, even in a worst-case scenario involving this litigation, the company’s potential is still strong. We are likely to see some volatility either positive or negative, depending on the outcome. If the decision goes against Liquidia, I believe the share price decline would be short-lived as their PAH business alone supports a higher stock price than today.
The company recently presented at the JP Morgan healthcare conference on January 14. They provided details on the launch of Yutrepia, the potential for their next generation nebulizer, L606, and a host of new clinical trials to show that Yutrepia can also effectively treat Pulmonary Fibrosis, Systemic Sclerosis associated with Raynaud’s Phenomenon, and COPD. These markets dwarf the current PAH and PH-ILD market that Liquidia participates in.
In the second half of 2026, Liquidia is participating in an investigator initiated study to confirm the effectiveness of combining sotatercept with treprostinil for better patient outcomes. Sotatercept is an injection that reduces lung vessel pressure, while inhaled treprostinil reduces long vessel resistance. This combination therapy has the potential to become the gold standard for PAH patients. The commercial name for sotatercept is Winrevair. It is owned by Merck. Merck has been active in the M&A space, and many believe that they would be a logical buyer of Liquidia.
At the JP Morgan conference, Roger Jeffs said that he believed Liquidia would reach $1 billion in revenue in 2027. I believe that number is conservative. In early 2024, I said that I thought Liquidia’s base case was $75 per share by 2027 with a bull case of $100 per share. If the company generates the $1 billion in revenue that Roger Jeffs believes, shares should trade above that bull case number.
ENDI Corp. (ENDI)
One year ago, I wrote that ENDI was the best near-term risk/reward opportunity since I launched Arquitos Capital. Shares began 2025 trading at $11.43, which was less than 5x EBITDA net of cash and investments. Assets Under Management (AUM) were at $3.4 billion and the annual EBITDA run rate was $8 million. ENDI’s stock was cheap on its own and did not give credit for any future growth. The chances of losing money on an investment with those characteristics and at that price were miniscule.
The stock rose to $16.75 by year end. The EBITDA run rate hit $12.5 million annually, and AUM stood at $4.1 billion. Using the last publicly reported financial numbers, ENDI continues to trade around 5x EBITDA. Similar specialized asset managers trade at valuations far above that number.
A major overhang for the stock was the outstanding warrants at the company level. These were issued when ENDI acquired CrossingBridge. The strike price was $8 per share. The accounting treatment for these warrants made the income statement confusing and especially difficult for new potential shareholders analyzing the company for the first time.
The company did an early cashless exercise of these warrants (at a discount) prior to the end of 2025. This means that the financial statements in the future will be more clear and the company will be easier to analyze. Now that the market cap is above $100 million and the structure is cleaner, I expect the company will begin to attract additional interest and become more fairly valued over time.
Finch Therapeutics (FNCH)
Finch shares ended 2025 at $13.49, rising by 19% for the year despite no major updates to their court case.
Finch won a jury trial in August 2024 against Ferring Pharmaceuticals, with the jury finding that Ferring infringed on three of Finch’s patents. The jury awarded Finch approximately $30 million in a one-time licensing fee and pre-trial interest, plus ongoing royalties to be determined by the judge and post-trial interest. Importantly, the jury also found willful infringement, opening the door for enhanced damages up to three times the jury award.
We continue to wait for the post trial decision from the judge where she will determine an ongoing royalty rate and determine whether enhanced damages will be awarded. My guess is that part of the delay is that the parties are attempting to negotiate a settlement. Patience is our friend here, as interest on the judgement accrues at the Federal Reserve discount rate plus 5%.
I expect our administrator to deliver the K-1s in late March.
For our audit, you may receive communications and verification requests from Wipfli as they perform their work. The sooner you complete and return these items, the sooner we can receive the fund’s final audit. I appreciate your cooperation. If you have any questions about any of these items, please let me know.
Thank you again your commitment to Arquitos, and best wishes for 2026!
Best regards,
Steven Kiel
Arquitos Capital
Read more hedge fund letters here


